How Many Electrons Are in Carbon's Valence Shell
Carbon has atomic number 6 which belongs to group 14 in the periodic table. The total number of electrons in a valence shell is called a valence electron.

How Many Valence Electrons Does Carbon Have Perfect Atom
Atomic carbon has six electrons.

. The noble gases and helium are all happy because their valence shell is full. Carbon typically shares electrons to achieve a complete valence shell forming bonds with multiple other atoms. The highest energy level of the carbon atom has two subshells.
For example carbon is in group 4 and has 4 valence electrons. Valence electrons in Neon Ne 8. A pair of electrons alone or an electron that is part of a covalent bond.
The highest-numbered shell is the third shell which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. Carbon needs four more valence electrons or a total of eight valence electrons to fill its outer energy. Valence electrons in Carbon is 4.
There is a point we should pay attention to here. Therefore the valence electrons of carbon are four. The main group number for an element can be found from its column on the periodic table.
Oxygen is in group 6 and has 6 valence electrons. Valence electrons in Sodium Na 1. 119 rows Valence electrons in Beryllium Be 2.
Carbon has 4 valence electrons on the valence shell. Valence electrons in Fluorine F 7. That gives a total of 5 electrons so neutral phosphorus atoms have 5 valence electrons.
Even though it only has two electrons it is still grouped with the noble gases that have eight electrons in their outermost orbitals. The outermost shell of. Valence electrons in Carbon C 4.
Carbon C as a group 14 element has four electrons in its outer shell. Two inner shell electrons core in the 1s orbital and four valence electrons outer shell in the 2s and 2p orbitals. How old is the queen.
Carbon has 4 valence electrons in the outermost shell. It is very stable with only two electrons in its outer orbital valence shell. It has 6 electrons distributed in 2 energy shells.
How many electrons does carbon atomic number 6 contain in its outer valence shell. The electronic configuration of carbon is 24 Carbon. How many electrons does carbon atomic number 6 contain in its outer valence shell.
6 b2 O c. This may interest you. The electron configuration of carbon shows that the last shell of carbon has four electrons2s 2 2p 2.
What kind of outer shell is an atom left with. An electron in the valence shell of an atom. Valence electrons in Nitrogen N 5.
Carbon atom has 4 valence electrons. Valence electrons in Oxygen O 6. 6 b2 O c.
Carbon has 4 valence electrons on the valence shell. Two inner shell core electrons in the 1s orbital and four valence outer most shell electrons in the 2s and 2p orbitals. Valence electrons in Magnesium Mg 2.
Atomic carbon has six electrons. What is the valence electron for carbon. As A Result the columns of the table of elements mirror the number of electrons located in each components valence shell which therefore creates how the.
For neutral atoms the number of valence electrons is equal to the atoms main group number. Because if you check electron configuration for carbon you will see that last shell is 2s2 2p2 which means that carbon has 4 valence electrons. The innermost shell of the carbon is fully occupied with 2 electrons in the 1s orbital while the next shell which is also the outermost shell gets partially filled with the.
Carbon C en masse 14 component has 4 electrons in its outershell Carbon generally shares electrons to attain a full valence shell generating bonds with many various various other atoms. A carbon atom has a total of 6 electrons revolving around the nucleus. Valence electrons in Boron B 3.
There are four electrons in the outer or valence shell of a neutral carbon atom. Valence electrons in Carbon. How many electrons does carbon need to stable.
The atomic number of carbon is 6 It is present in group 4 of the Periodic Table of Elements.
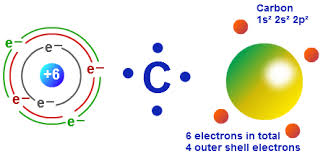
Carbon Valence Electrons Carbon Valency C With Dot Diagram

How Many Electrons Are In Carbon Quora
Comments
Post a Comment